Ozanimod
 Data Sheet
For research use only. Not for human use.
Data Sheet
For research use only. Not for human use.
* Required Fields.
Please complete the form below and you will get the price list in 1 minute.
* Required Fields.
Please complete the form below and we will contact you shortly.
* Required Fields.
Please complete the form below and we will contact you shortly.

| CAS No. | 1306760-87-1 | Cat. No. | BCP16513 |
| Related Products | 1618636-37-5(Ozanimod hydrochloride) | ||
| Name | Ozanimod | ||
| Synonyms | RPC1063; RPC-1063; RPC 1063; | ||
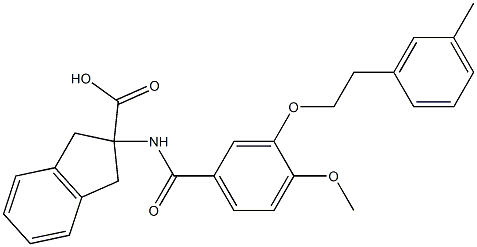
| SMILES | |||
| Chemical Name | |||
| Formula | C23H24N4O3 | M. Wt | 404.46 |
| Purity | 98% | Storage | Store at 4-8°C |
| Description | Target: sphingosine 1-phosphate 1 and 5 receptor Ozanimod represents a potential franchise in immunology. Ozanimod is the potential next-to-market oral agent for the treatment of relapsing multiple sclerosis. In a Phase 2 trial in patients with RMS, Ozanimod achieved the primary endpoint of reduction in MRI brain lesion activity as well as secondary endpoints measuring effects on other MRI parameters. The overall safety profile of Ozanimod is consistent with the results of prior trials and continues to demonstrate differentiation against other oral agents for treatment of RMS. Ozanimod is also being studied in inflammatory bowel disease (IBD). The TOUCHSTONE Phase 2 trial of Ozanimod in UC met its primary endpoint and all secondary endpoints with statistical significance in patients on the 1.0 mg dose of Ozanimod in the 8-week induction period. The overall safety and tolerability profile of Ozanimod is consistent with the results of the recent Phase 2 trial in RMS, and continues to sup | ||
Recommend Products
More >
-
-
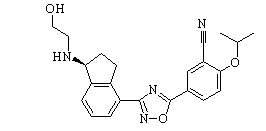
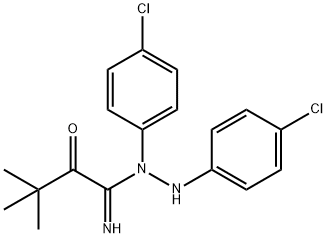
BMS-986278
Cat. No.:BCP45657
No.:2170126-74-4
Product Details -
-

-
-
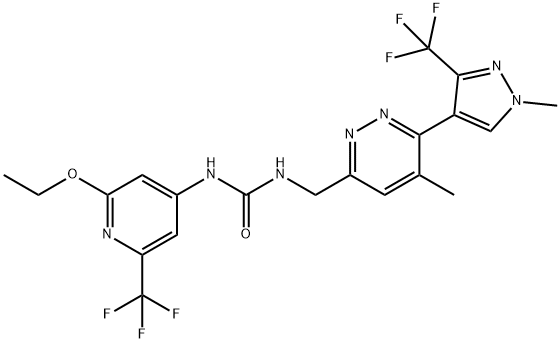
S1P1 Agonist III
Cat. No.:BCP41026
No.:1324003-64-6
Product Details -
PF429242 dihydrochloride
Cat. No.:BCP39849
No.:2248666-66-0
Product Details -
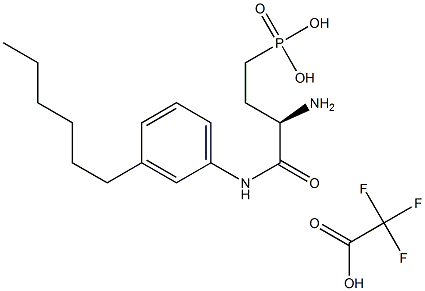
W146 trifluoroacetate salt
Cat. No.:BCP38982
No.:909725-62-8
Product Details -
-
-
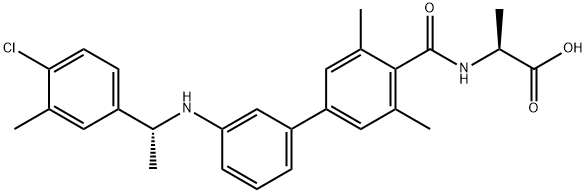
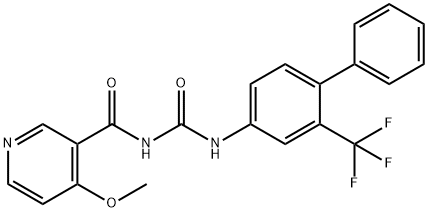
NIBR 0213
Cat. No.:BCP37856
No.:1233332-14-3
Product Details -
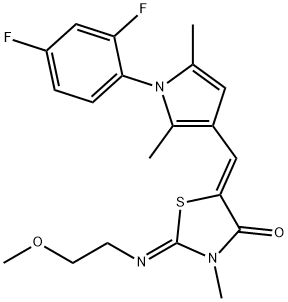
-
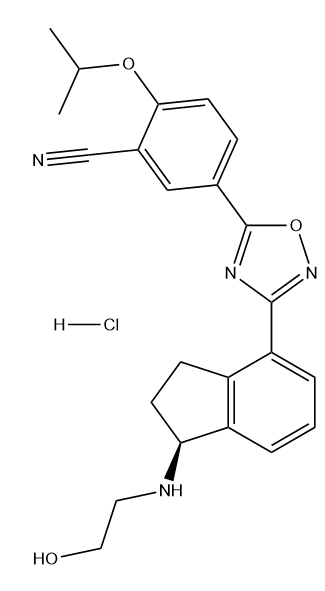
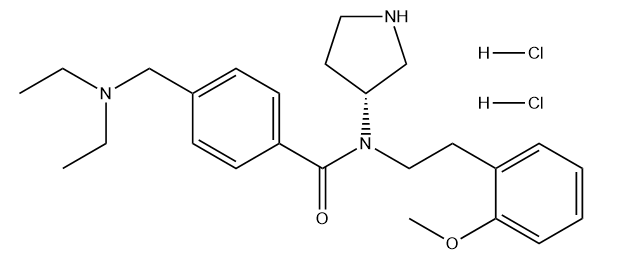
Ozanimod hydrochloride
Cat. No.:BCP37091
No.:1618636-37-5
Product Details -
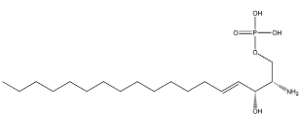
Sphingosine 1-phosphate
Cat. No.:BCP36245
No.:26993-30-6
Product Details -
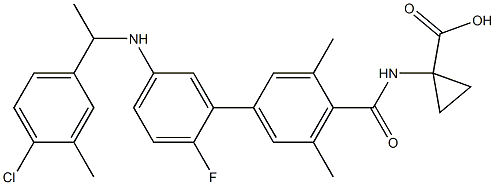
-
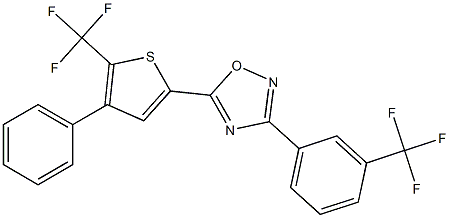
-
SAR-100842
Cat. No.:BCP34736
No.:1195941-38-8
Product Details -
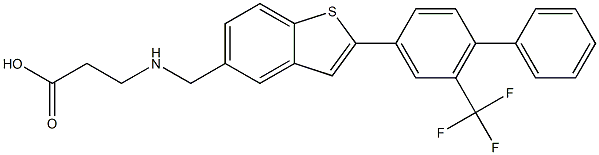
-
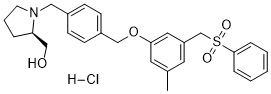
PF-543 HCl
Cat. No.:BCP26198
No.:1706522-79-3
Product Details -
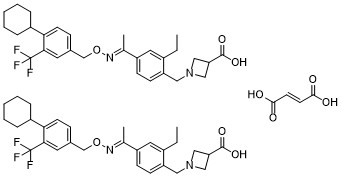
Siponimod fumarate
Cat. No.:BCP25217
No.:1234627-85-0
Product Details

Tags:Ozanimod supplier,Ozanimod purchase,Ozanimod manufacturer,Ozanimod distributor,Ozanimod cost,Ozanimod buy,Ozanimod for sale