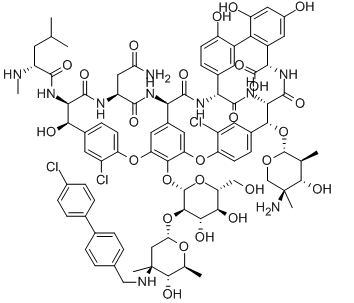
Oritavancin
 Data Sheet
For research use only. Not for human use.
Data Sheet
For research use only. Not for human use.
* Required Fields.
Please complete the form below and you will get the price list in 1 minute.
* Required Fields.
Please complete the form below and we will contact you shortly.
* Required Fields.
Please complete the form below and we will contact you shortly.

| CAS No. | 171099-57-3 | Cat. No. | BCP15378 |
| Name | Oritavancin | ||
| Synonyms | LY333328; LY-333328; LY 333328; | ||
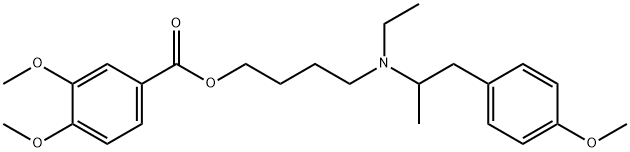
| SMILES | CC1C(C(CC(O1)OC2C3C(=O)NC(C4=CC(=CC(=C4C5=C(C=CC(=C5)C(C(=O)N3)NC(=O)C6C7=CC(=C(C(=C7)OC8=C(C=C(C=C8)C(C(C(=O)NC(C(=O)N6)CC(=O)N)NC(=O)C(CC(C)C)NC)O)Cl)OC9C(C(C(C(O9)CO)O)O)OC1CC(C(C(O1)C)O)(C)NCC1=CC | ||
| Chemical Name | |||
| Formula | C86H97Cl3N10O26 | M. Wt | 1793.1 |
| Purity | 98% | Storage | Store at 4-8°C |
| Description | Oritavancin, also known as LY333328, is a novel semisynthetic glycopeptide antibiotics. On August 6, 2014, the FDA approved oritavancin for treatment of skin infections. Oritavancin possesses potent and rapid bactericidal activity in vitro against a broad spectrum of both resistant and susceptible Gram-positive bacteria, including Staphylococcus aureus, MRSA, enterococci, and streptococci. Oritavancin was more active than either metronidazole or vancomycin against strains of Clostridium difficile tested. Oritavancin has potential use as a therapy for exposure to Bacillus anthracis, the Gram-positive bacterium that causes anthrax, having demonstrated efficacy in a mouse model both before and after exposure to the bacterium. The 4'-chlorobiphenylmethyl group disrupts the cell membrane of Gram-positive bacteria. It also acts by inhibition of transglycosylation and inhibition of transpeptidation. | ||
Recommend Products
More >
-
-
-
-
-
-
-
-
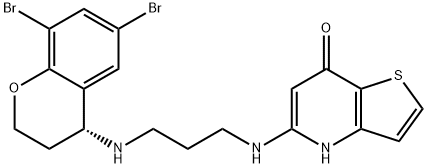
-
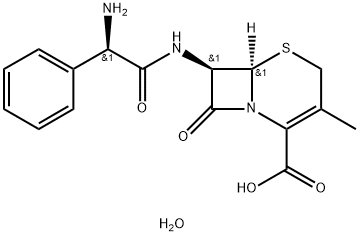
Cephalexin monohydrate
Cat. No.:BCP49078
No.:23325-78-2
Product Details -
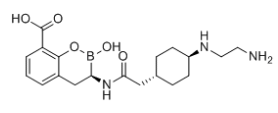
Taniborbactam
Cat. No.:BCP36483
No.:1613267-49-4
Product Details -
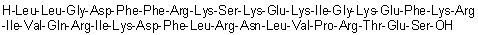
Cathelicidin LL 37 (human)
Cat. No.:BCP30243
No.:154947-66-7
Product Details -
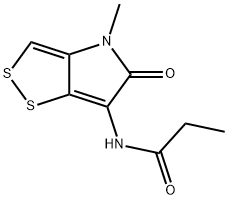
-
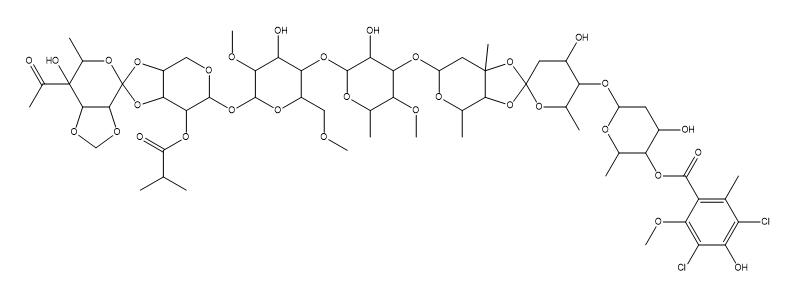
-
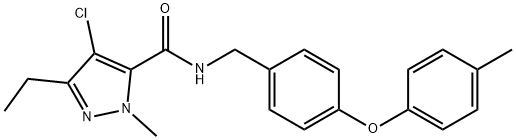
Tolfenpyrad
Cat. No.:BCP24108
No.:129558-76-5
Product Details -
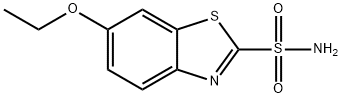
Ethoxzolamide
Cat. No.:BCP24088
No.:452-35-7
Product Details -
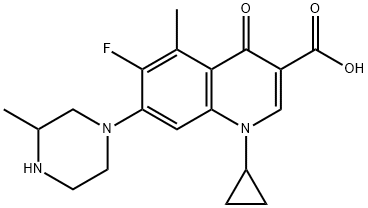
Grepafloxacin
Cat. No.:BCP23880
No.:119914-60-2
Product Details -
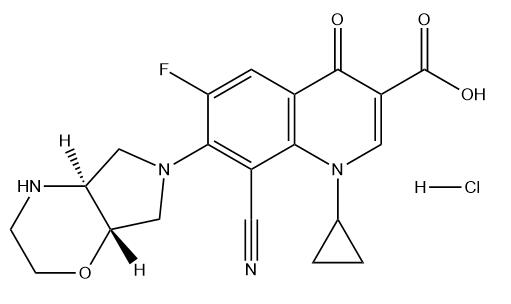
Finafloxacin Hydrochloride
Cat. No.:BCP23879
No.:209342-41-6
Product Details -
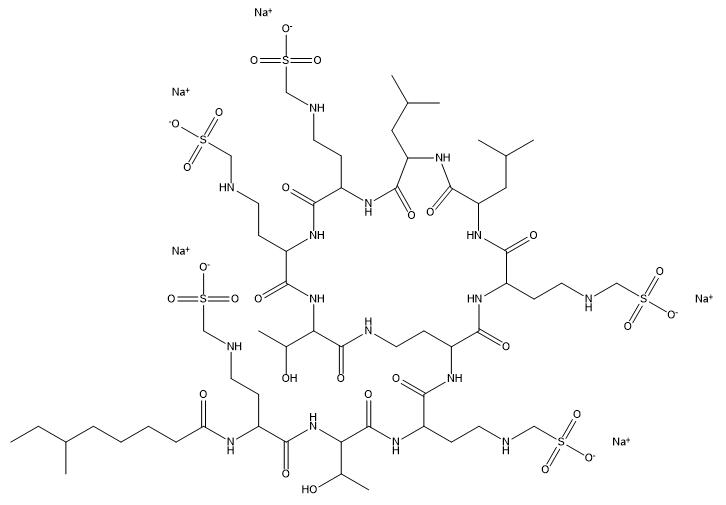
Colistimethate Sodium
Cat. No.:BCP15947
No.:8068-37-9
Product Details -
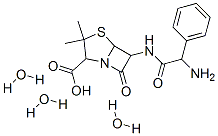
Ampicillin trihydrate
Cat. No.:BCP28578
No.:7177-48-2
Product Details -

Tags:Oritavancin supplier,Oritavancin purchase,Oritavancin manufacturer,Oritavancin distributor,Oritavancin cost,Oritavancin buy,Oritavancin for sale





