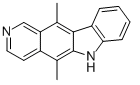
Ellipticine
 Data Sheet
For research use only. Not for human use.
Data Sheet
For research use only. Not for human use.
* Required Fields.
Please complete the form below and you will get the price list in 1 minute.
* Required Fields.
Please complete the form below and we will contact you shortly.
* Required Fields.
Please complete the form below and we will contact you shortly.

| CAS No. | 519-23-3 | Cat. No. | BCP13208 |
| Name | Ellipticine | ||
| Synonyms | CCG-36483; CCG36483; CCG 36483; DB-052047; K00071; LP00531; LS-133282; NSC 71795; NSC71795; NSC-71795; TCMDC-125546; | ||
| SMILES | |||
| Chemical Name | |||
| Formula | C17H14N2 | M. Wt | 246.31 |
| Purity | 98% | Storage | Store at 4-8°C |
| Description | Ellipticine is a DNA intercalating agent and a DNA topoisomerase II inhibitor. Ellipticine is also a natural product, isolated in 1959 from the Australian evergreen tree of the Apocynaceae family. Ellipticine was found to be an extremely promising anticancer drug. The planar polycyclic structure was found to interact with DNA through intercalation, exhibiting a high DNA binding affinity (10(6) M(-1)). The presence of protonatable ring nitrogens distinguished ellipticine from other simple intercalators. Both monocationic and uncharged species were found to be present under physiological conditions. The positive charge stabilized the binding of ellipticine to nucleic acids, while the more lipophilic uncharged compound was shown to readily penetrate membrane barriers. The structural nature of these compounds offers a plausible basis for the implication of multiple modes of action, including DNA binding, interactions with membrane barriers, oxidative bioactivation and modification of enzyme function; most notably that of topoisomerase II and telomerase. | ||
Recommend Products
More >
-

Fortunellin
Cat. No.:BCP39988
No.:20633-93-6
Product Details -
-
-
-
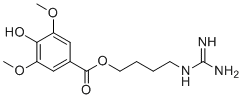
-
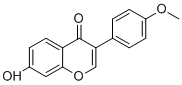
-
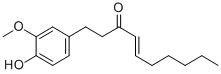
-
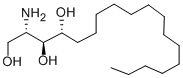
Phytosphingosine
Cat. No.:BCP23610
No.:554-62-1
Product Details -
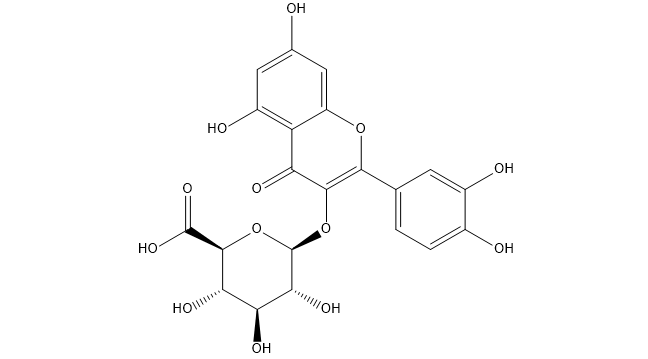
Miquelianin
Cat. No.:BCP20427
No.:22688-79-5
Product Details -
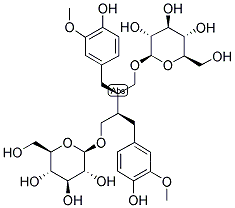
Seco-isolariciresinol diglucoside
Cat. No.:BCP25486
No.:158932-33-3
Product Details -
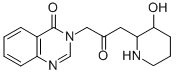
Febrifugine
Cat. No.:BCP15889
No.:24159-07-7
Product Details -
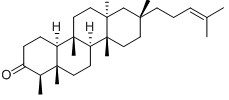
-
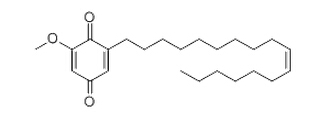
Irisquinone
Cat. No.:BCP13285
No.:56495-82-0
Product Details -
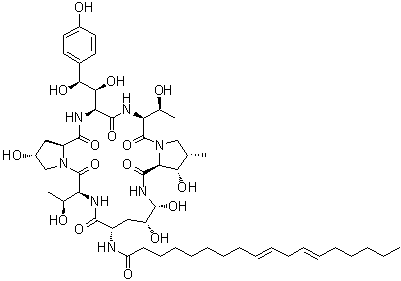
Echinocandin B
Cat. No.:BCP12574
No.:54651-05-7
Product Details -
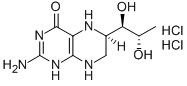
Sapropterin dihydrochloride
Cat. No.:BCP09678
No.:69056-38-8
Product Details -
-
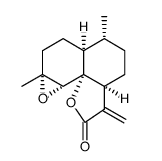
Arteannuin B
Cat. No.:BCP27596
No.:50906-56-4
Product Details -
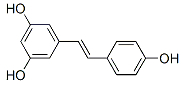
-
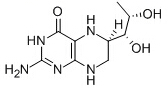
Sapropterin
Cat. No.:BCP03735
No.:62989-33-7
Product Details -

Tags:Ellipticine supplier,Ellipticine purchase,Ellipticine manufacturer,Ellipticine distributor,Ellipticine cost,Ellipticine buy,Ellipticine for sale




