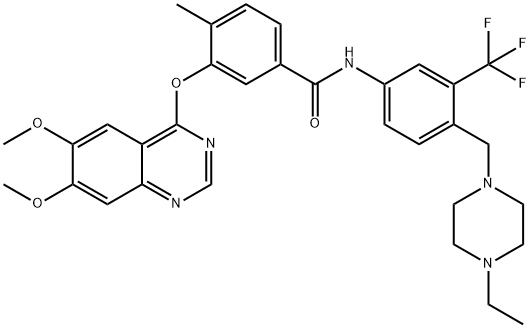
Bosutinib Monohydrate
 Data Sheet
For research use only. Not for human use.
Data Sheet
For research use only. Not for human use.
* Required Fields.
Please complete the form below and you will get the price list in 1 minute.
* Required Fields.
Please complete the form below and we will contact you shortly.
* Required Fields.
Please complete the form below and we will contact you shortly.

| CAS No. | 918639-08-4 | Cat. No. | BCP17036 |
| Name | Bosutinib Monohydrate | ||
| Synonyms | SKI606; SKI 606; SK-I606; | ||
| SMILES | |||
| Chemical Name | |||
| Formula | C26H31Cl2N5O4 | M. Wt | 548.46 |
| Purity | 98% | Storage | Store at 4-8°C |
| Description | Bosutinib, also known as SKI-606, is a synthetic quinolone derivative and dual kinase inhibitor that targets both Abl and Src kinases with potential antineoplastic activity. Unlike imatinib, bosutinib inhibits the autophosphorylation of both Abl and Src kinases, resulting in inhibition of cell growth and apoptosis. Because of the dual mechanism of action, this agent may have activity in resistant CML disease, other myeloid malignancies and solid tumors. Abl kinase is upregulated in the presence of the abnormal Bcr-abl fusion protein which is commonly associated with chronic myeloid leukemia (CML). Overexpression of specific Src kinases is also associated with the imatinib-resistant CML phenotype. Bosutinib received US FDA and EU European Medicines Agency approval on September 4, 2012 and 27 March, 2013 respectively for the treatment of adult patients with Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML) with resistance, or intolerance to prior therapy. | ||
Recommend Products
More >
-
-
-
Vodobatinib
Cat. No.:BCP43522
No.:1388803-90-4
Product Details -
Elzovantinib
Cat. No.:BCP40929
No.:2271119-26-5
Product Details -
-
DGY-06-116
Cat. No.:BCP37659
No.:2556836-50-9
Product Details -
PD173955-Analog1
Cat. No.:BCP36116
No.:185039-99-0
Product Details -
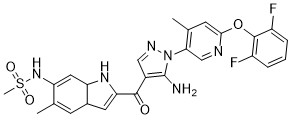
CH6953755
Cat. No.:BCP35548
No.:2055918-71-1
Product Details -
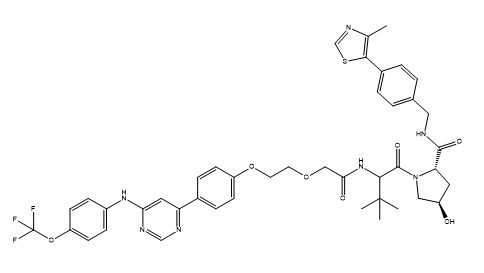
-
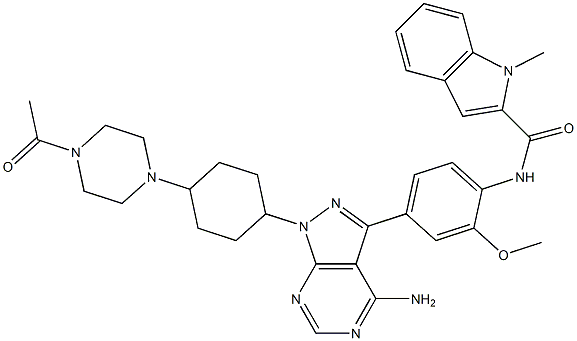
-
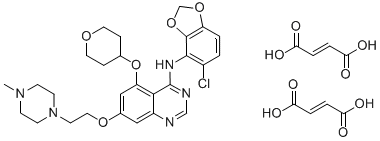
Saracatinib difumarate
Cat. No.:BCP25711
No.:893428-72-3
Product Details -
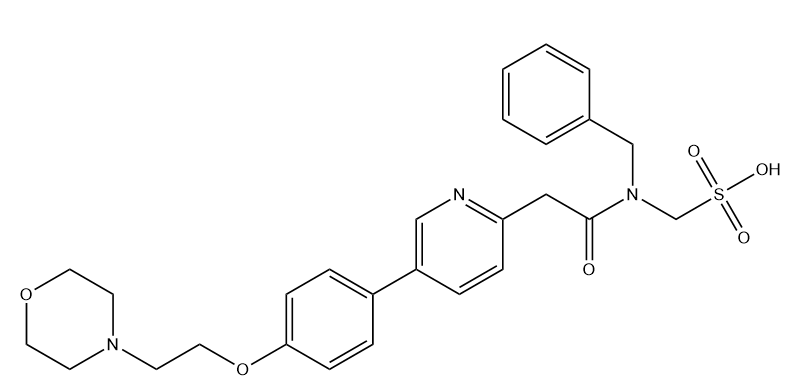
KX2-391 Mesylate
Cat. No.:BCP25173
No.:1080645-95-9
Product Details -
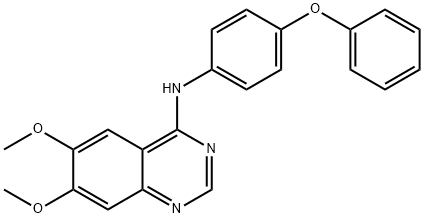
Src Kinase Inhibitor I
Cat. No.:BCP24909
No.:179248-59-0
Product Details -
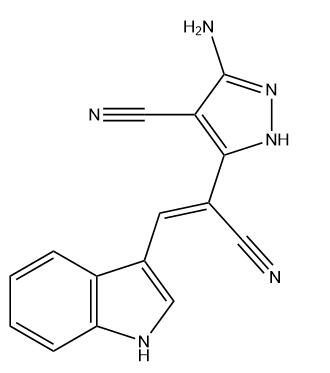
Tyrphostin AG 1112
Cat. No.:BCP24145
No.:153150-84-6
Product Details -
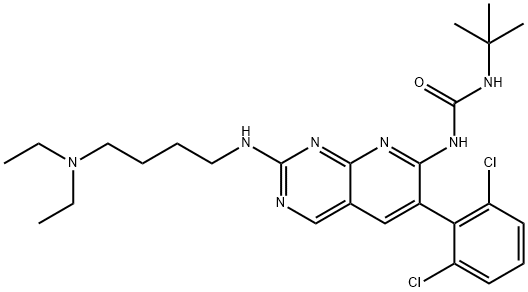
-
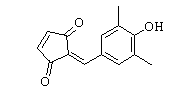
-
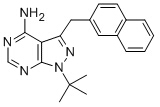
-
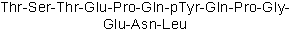
pp60c-src Fragment 521-533
Cat. No.:BCP27682
No.:149299-77-4
Product Details -
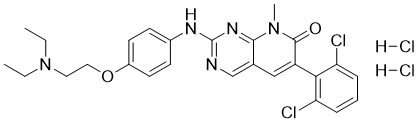
PD 166285 dihydrochloride
Cat. No.:BCP22349
No.:212391-63-4
Product Details -
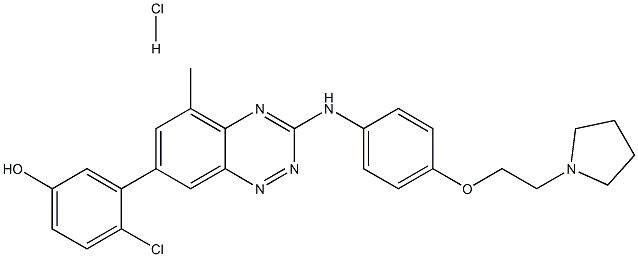
TG 100572 Hydrochloride
Cat. No.:BCP21364
No.:867331-64-4
Product Details

Tags:Bosutinib Monohydrate supplier,Bosutinib Monohydrate purchase,Bosutinib Monohydrate manufacturer,Bosutinib Monohydrate distributor,Bosutinib Monohydrate cost,Bosutinib Monohydrate buy,Bosutinib Monohydrate for sale