埃替拉韦
| CAS号 | 697761-98-1 | 货号 | BCP01801 |
| 中文名 | 埃替拉韦 | ||
| 英文名 | Elvitegravir | ||
| 中文别名 | 埃替格韦; | ||
| 英文别名 | GS-9137; GS 9137; GS9137; EVG; JTK 303; JTK-303; JTK303;D06677; | ||
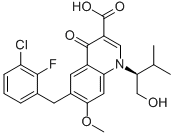
| 分子式 | C23H23ClFNO5 | 分子量 | 447.88 |
| 生物活性 | Elvitegravir is a human immunodeficiency virus type 1 (HIV-1) integrase strand transfer inhibitor (INSTI) used for the treatment of HIV-1 infection in antiretroviral treatment-experienced adults. Because integrase is necessary for viral replication, inhibition prevents the integration of HIV-1 DNA into the host genome and thereby blocks the formation of the HIV-1 provirus and resulting propagation of the viral infection. Although available as a single dose tablet, elvitegravir must be used in combination with an HIV protease inhibitor coadministered with ritonavir and another antiretroviral drug. Elvitegravir was first licensed from Japan Tobacco in 2008 and developed by Gilead Sciences. It was FDA approved on August 27, 2012. On September 24, 2014, the FDA approved the single pill form of elvitegravir. | ||
| 信号通路 | Protease/Metabolic Enzyme Microbiology/Virology | ||
| 靶 点 | Integrase HIV | ||
结构式
本页面部分数据来自于公开的网络资源,所以瀚香生物不能保证其准确性。
不同批次的产品详情请咨询我们的客服:
- 全国免费电话:400-099-8200
瀚香生物(Biochempartner) 的所有产品和服务仅用于科学研究,禁止用于人体和治疗!
