即时询价 ×
* 必填项
请填妥以下表格,您将在一分钟内得到邮件机器人给你的报价。
Inquiry Online
×
* Required Fields.
Please complete the form below and we will contact you shortly.
Bulk Inquiry
×
* Required Fields.
Please complete the form below and we will contact you shortly.

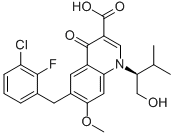
| CAS号 | 697761-98-1 | 货号 | BCP01801 |
| 中文名 | 埃替拉韦 | ||
| 英文名 | Elvitegravir | ||
| 中文别名 | 埃替格韦; | ||
| 英文别名 | GS-9137; GS 9137; GS9137; EVG; JTK 303; JTK-303; JTK303;D06677; | ||
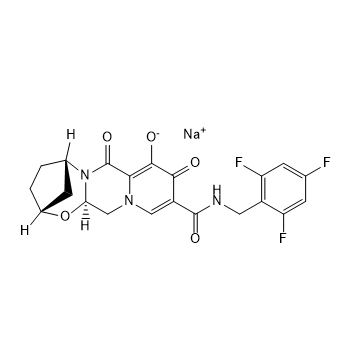
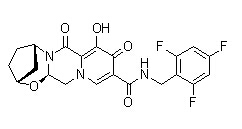
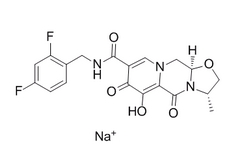
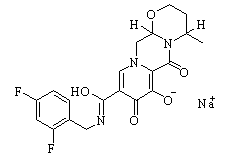
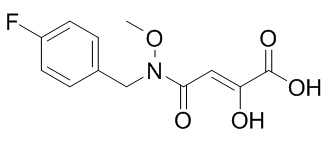
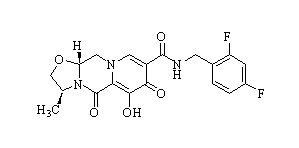
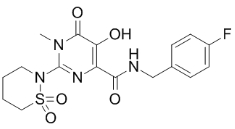
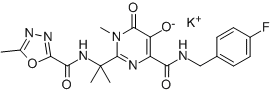
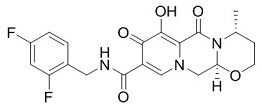
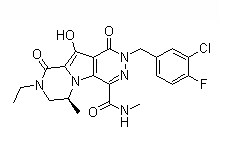
| SMILES | CC(C)C(CO)N1C=C(C(=O)C2=C1C=C(C(=C2)CC3=C(C(=CC=C3)Cl)F)OC)C(=O)O | ||
| 化学名称 | |||
| 分子式 | C23H23ClFNO5 | 分子量 | 447.88 |
| 纯度 | 98% | 配送 | 惯例下常温包邮 |
| 产品描述 | Elvitegravir is a human immunodeficiency virus type 1 (HIV-1) integrase strand transfer inhibitor (INSTI) used for the treatment of HIV-1 infection in antiretroviral treatment-experienced adults. Because integrase is necessary for viral replication, inhibition prevents the integration of HIV-1 DNA into the host genome and thereby blocks the formation of the HIV-1 provirus and resulting propagation of the viral infection. Although available as a single dose tablet, elvitegravir must be used in combination with an HIV protease inhibitor coadministered with ritonavir and another antiretroviral drug. Elvitegravir was first licensed from Japan Tobacco in 2008 and developed by Gilead Sciences. It was FDA approved on August 27, 2012. On September 24, 2014, the FDA approved the single pill form of elvitegravir. | ||
相关产品推荐
查看更多 >Tags:Elvitegravir 供应商,Elvitegravir 购买,Elvitegravir 生产,Elvitegravir 批量,Elvitegravir 供应,Elvitegravir 订购,Elvitegravir 采购





 参考资料
参考资料