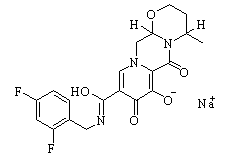
Dolutegravir sodium New
 Data Sheet
For research use only. Not for human use.
Data Sheet
For research use only. Not for human use.
* Required Fields.
Please complete the form below and you will get the price list in 1 minute.
* Required Fields.
Please complete the form below and we will contact you shortly.
* Required Fields.
Please complete the form below and we will contact you shortly.

| CAS No. | 1051375-19-9 | Cat. No. | BCP11890 |
| Name | Dolutegravir sodium | ||
| Synonyms | Dolutegravir sodium salt;S/GSK1349572 sodium;GSK 1349572A;GSK-1349572A;GSK1349572A; | ||
| SMILES | |||
| Chemical Name | |||
| Formula | C20H18F2N3NaO5 | M. Wt | 441.36 |
| Purity | 98% | Storage | Store at 4-8°C |
| Description | Dolutegravir (DTG, GSK1349572) is metabolized primarily by uridine diphosphate glucuronyltransferase (UGT)1A1, with a minor role of cytochrome P450 (CYP)3A, and with renal elimination of unchanged drug being extremely low (< 1% of the dose). Fifty-three percent of the total oral dose is excreted unchanged in the feces but it is unknown if all or part of this is due to unabsorbed drug or some percentage of biliary excretion of the glucuronide conjugate which can be further degraded to form the parent compound in the gut lumen. The current Food and Drug Administration (FDA) draft guidance for renal impairment studies states that a pharmacokinetic (PK) study in patients with renal impairment should be conducted even for those drugs primarily metabolized or secreted in bile, because renal impairment can inhibit some pathways of hepatic and gut drug metabolism and transport. | ||
Recommend Products
More >
-
-
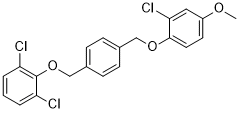
GSK2200150A
Cat. No.:BCP29624
No.:1443138-53-1
Product Details -
-
-
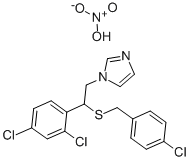
Sulconazole nitrate
Cat. No.:BCP25551
No.:61318-91-0
Product Details -
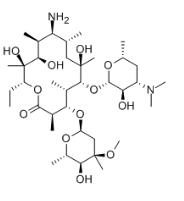
Erythromycylamine
Cat. No.:BCP20223
No.:26116-56-3
Product Details -
-
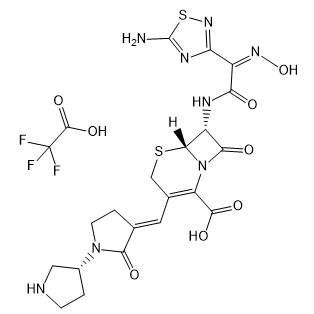
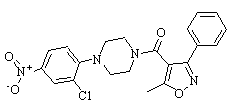
Ceftobiprole trifluoroacetate
Cat. No.:BCP19977
No.:1031275-04-3
Product Details -
-
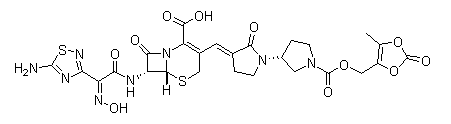
Ceftobiprole medocaril
Cat. No.:BCP18211
No.:376653-43-9
Product Details -
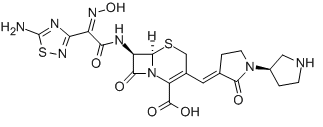
Ceftobiprole
Cat. No.:BCP18210
No.:209467-52-7
Product Details -
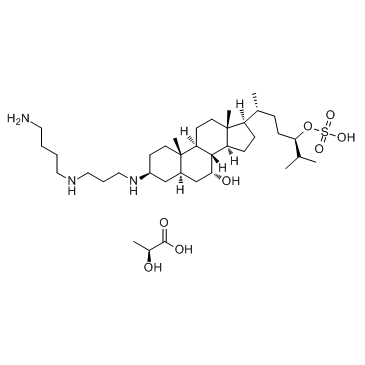
Squalamine lactate
Cat. No.:BCP24210
No.:320725-47-1
Product Details -
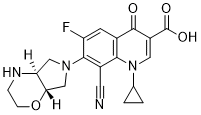
Finafloxacin
Cat. No.:BCP23882
No.:209342-40-5
Product Details -
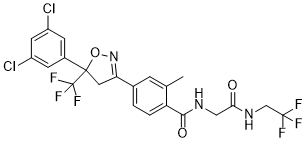
Fluralaner
Cat. No.:BCP23785
No.:864731-61-3
Product Details -
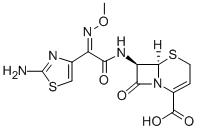
Ceftizoxime
Cat. No.:BCP12173
No.:68401-81-0
Product Details -
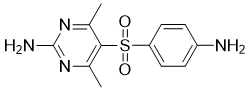
Sulfamethazine
Cat. No.:BCP28439
No.:57-68-1
Product Details -
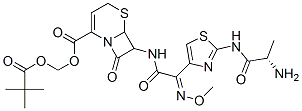
Ceftizoxime alapivoxil
Cat. No.:BCP28430
No.:135821-54-4
Product Details -
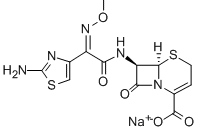
Ceftizoxime sodium
Cat. No.:BCP11978
No.:68401-82-1
Product Details -
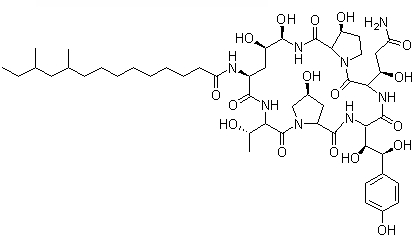
Pneumocandin B0
Cat. No.:BCP09296
No.:135575-42-7
Product Details -
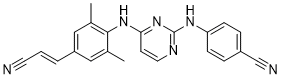
Rilpivirine
Cat. No.:BCP03563
No.:500287-72-9
Product Details

Tags:Dolutegravir sodium supplier,Dolutegravir sodium purchase,Dolutegravir sodium manufacturer,Dolutegravir sodium distributor,Dolutegravir sodium cost,Dolutegravir sodium buy,Dolutegravir sodium for sale