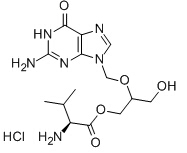
Valganciclovir hydrochloride
 Data Sheet
For research use only. Not for human use.
Data Sheet
For research use only. Not for human use.
* Required Fields.
Please complete the form below and you will get the price list in 1 minute.
* Required Fields.
Please complete the form below and we will contact you shortly.
* Required Fields.
Please complete the form below and we will contact you shortly.

| CAS No. | 175865-59-5 | Cat. No. | BCP08146 |
| Name | Valganciclovir hydrochloride | ||
| Synonyms | |||
| SMILES | |||
| Chemical Name | |||
| Formula | C14H23ClN6O5 | M. Wt | 390.83 |
| Purity | 98% | Storage | Store at 4-8°C |
| Description | in vitro: In cell culture model systems using Caco-2 cells for PEPT1 and SKPT cells for PEPT2, valganciclovir inhibited glycylsarcosine transport mediated by PEPT1 and PEPT2 with K(i) values (inhibition constant) of 1.68+/-0.30 and 0.043+/- 0.005 mM, respectively. The inhibition by valganciclovir was competitive in both cases . in vivo: 37 patients were enrolled; 19 patients received treatment with VGV and 18 patients received treatment with GCV. The VGV was not inferior in efficacy to GCV as pre-emptive therapy, with rates of viral clearance at 28 days of 89.5% and 83%, respectively (P-value for non-inferiority = 0.030). Toxicities were similar between the 2 arms. No patients developed CMV disease [2]. Patients being treated with an alemtuzumab-containing regimen received prophylaxis with either valaciclovir 500 mg orally daily orvalganciclovir 450 mg orally twice daily. None of the 20 patients randomized to valganciclovir experienced CMV reactivation (P = .004) . | ||
Recommend Products
More >
-
-
GSK2200150A
Cat. No.:BCP29624
No.:1443138-53-1
Product Details -
-
-
Sulconazole nitrate
Cat. No.:BCP25551
No.:61318-91-0
Product Details -
Erythromycylamine
Cat. No.:BCP20223
No.:26116-56-3
Product Details -
-
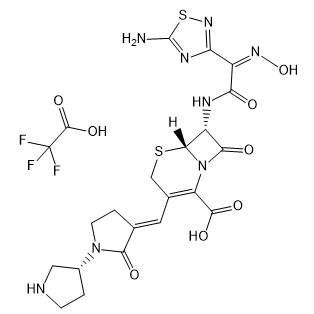
Ceftobiprole trifluoroacetate
Cat. No.:BCP19977
No.:1031275-04-3
Product Details -
-
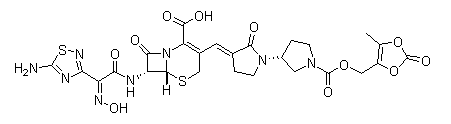
Ceftobiprole medocaril
Cat. No.:BCP18211
No.:376653-43-9
Product Details -
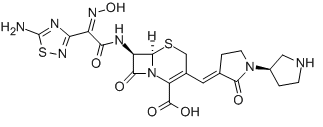
Ceftobiprole
Cat. No.:BCP18210
No.:209467-52-7
Product Details -
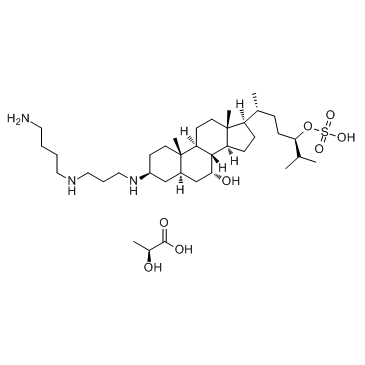
Squalamine lactate
Cat. No.:BCP24210
No.:320725-47-1
Product Details -
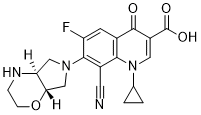
Finafloxacin
Cat. No.:BCP23882
No.:209342-40-5
Product Details -
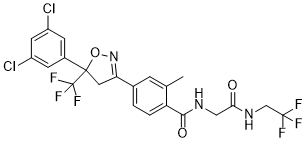
Fluralaner
Cat. No.:BCP23785
No.:864731-61-3
Product Details -
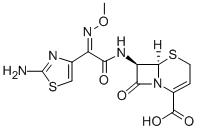
Ceftizoxime
Cat. No.:BCP12173
No.:68401-81-0
Product Details -
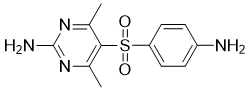
Sulfamethazine
Cat. No.:BCP28439
No.:57-68-1
Product Details -
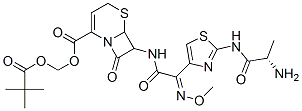
Ceftizoxime alapivoxil
Cat. No.:BCP28430
No.:135821-54-4
Product Details -
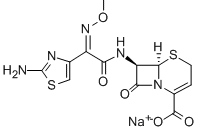
Ceftizoxime sodium
Cat. No.:BCP11978
No.:68401-82-1
Product Details -
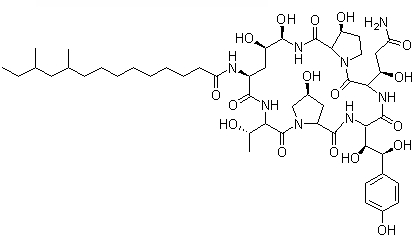
Pneumocandin B0
Cat. No.:BCP09296
No.:135575-42-7
Product Details -
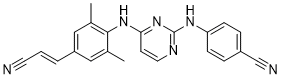
Rilpivirine
Cat. No.:BCP03563
No.:500287-72-9
Product Details

Tags:Valganciclovir hydrochloride supplier,Valganciclovir hydrochloride purchase,Valganciclovir hydrochloride manufacturer,Valganciclovir hydrochloride distributor,Valganciclovir hydrochloride cost,Valganciclovir hydrochloride buy,Valganciclovir hydrochloride for sale