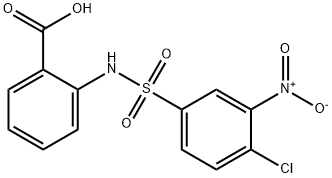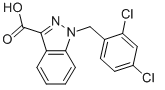
Lonidamine
 Data Sheet
For research use only. Not for human use.
Data Sheet
For research use only. Not for human use.
* Required Fields.
Please complete the form below and you will get the price list in 1 minute.
* Required Fields.
Please complete the form below and we will contact you shortly.
* Required Fields.
Please complete the form below and we will contact you shortly.

| CAS No. | 50264-69-2 | Cat. No. | BCP06555 |
| Name | Lonidamine | ||
| Synonyms | DICA;Diclondazolic Acid;AF1890;AF 1890;AF-1890; | ||
| SMILES | |||
| Chemical Name | |||
| Formula | C15H10Cl2N2O2 | M. Wt | 321.16 |
| Purity | 98% | Storage | Store at 4-8°C |
| Description | Lonidamine inhibits glycolysis by the inactivation of hexokinase. Interestingly, Lonidamine seems to enhance aerobic glycolysis in normal cells, but suppress glycolysis in cancer cells. This is most likely through the inhibition of the mitochondrially bound hexokinase. Lonidamine inhibits both respiration and glycolysis leading to a decrease in cellular ATP. In addition, lonidamine may increase programmed cell death. This stems from the observation that mitochondria and mitochondria-bound hexokinase are crucial for induction of apoptosis. In vitro models with lonidamine exhibit the hallmarks of apoptosis, including mitochondrial membrane depolarization, release of cytochrome C, phosphatidylserine externalization, and DNA fragmentation. Lonidamine also blocks CFTR Cl- channels in vitro. | ||
Recommend Products
More >
-

Ninerafaxstat 3HCl
Cat. No.:BCP49659
No.:2311824-72-1
Product Details -
-
-
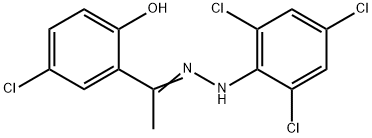
Mitochondrial fusion promoter M1
Cat. No.:BCP48945
No.:219315-22-7
Product Details -
-
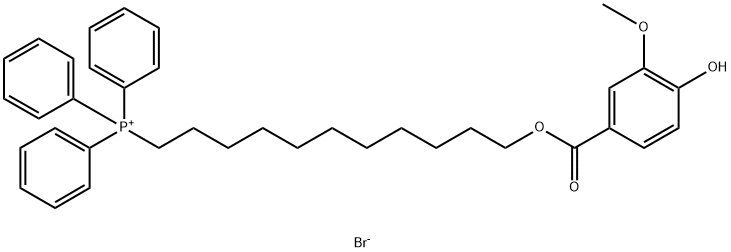
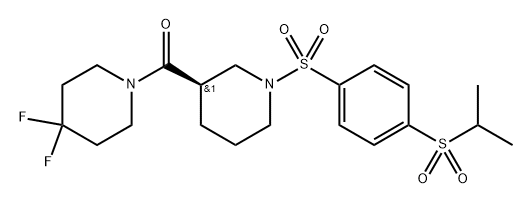
Mito-apocynin (C11)
Cat. No.:BCP46038
No.:1254044-38-6
Product Details -
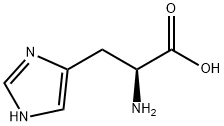
-
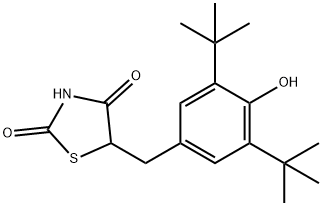
-
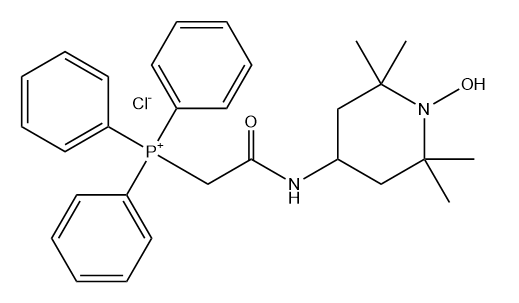
MitoTEMPO
Cat. No.:BCP43473
No.:1334850-99-5
Product Details -
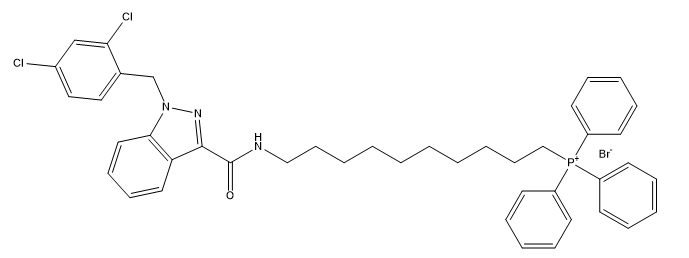
-
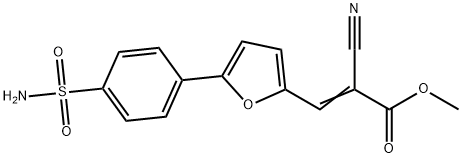
-
-
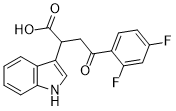
Mitochonic Acid 5
Cat. No.:BCP34060
No.:1354707-41-7
Product Details -
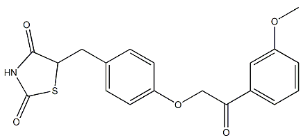
MSDC 0602
Cat. No.:BCP33206
No.:1133819-87-0
Product Details -
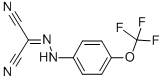
-
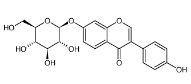
-
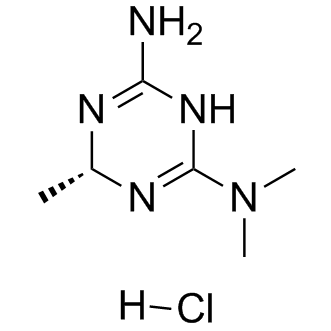
Imeglimin hydrochloride
Cat. No.:BCP11078
No.:775351-61-6
Product Details -
-

-

Tags:Lonidamine supplier,Lonidamine purchase,Lonidamine manufacturer,Lonidamine distributor,Lonidamine cost,Lonidamine buy,Lonidamine for sale